Ferroxyl test kit for free Iron
     [check ratings] [check ratings]
This is a highly sensitive test method for a local check of iron residues on stainless steel surfaces outlined in ASTM A 380-13, section 7.3.4.
The Ferroxyl test does not prove the existence of the passive layer - only the absence of Iron contamination. (see Copper Sulphate test or Passivity meter)
Testing should only be carried out by skilled personnel familiar with its limitations & hazards.
The pictures above show a Pass and a Fail so skill is required to interpret the results!
It is not recommended for use on products destined for use in food, medical or pharmaceutical unless complete removal of the cyanide solution can be guaranteed. It is possible to use our Copper Sulphate test kit to achieve a similar reaction but with less hazardous chemicals.
Carbon steel will only contaminate the stainless steel if the passive layer of chromium is destroyed. Very few fabricators have the capability to fabricate in an operating room environment. There are many ways to remove free iron and free iron oxides by using for example, Antox 95E (removal of contamination and passivation solution).
A ferroxyl test is a test done only to "detect" contamination. Once contamination is detected, then a removal procedure is carried out.
Due to the contents of this test kit we cannot ship outside the UK!
[ click here to view file attachment] click here to view file attachment]
|
 13 items
13 items 8 items
8 items 16 items
16 items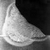 8 items
8 items 12 items
12 items 3 items
3 items 5 items
5 items 7 items
7 items 1 items
1 items 4 items
4 items 4 items
4 items 1 items
1 items 4 items
4 items 1 items
1 items
 £0
£0 Free delivery to UK mainland. Other areas may have additional costs. We have local agents covering Northern Ireland & Eire. Please contact us for more details.
Free delivery to UK mainland. Other areas may have additional costs. We have local agents covering Northern Ireland & Eire. Please contact us for more details. 









